The U.S. Food and Drug Administration (FDA) on Thursday announced its approval of Ixchiq, the first chikungunya vaccine.
The vaccine, which is made by Valneva, is approved for anyone age 18 and older who has a risk of being exposed to the virus.
The chikungunya virus is transmitted to people through bites from infected mosquitoes.
CHILDHOOD VACCINATIONS ARE AT AN ALL-TIME LOW, THE CDC REVEALS
“This virus is in a similar category as dengue or Zika and is carried by the same mosquitoes,” noted Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor.
The FDA described chikungunya as an “emerging global health threat,” with at least five million cases reported over the past 15 years.
The chikungunya virus is transmitted to people through bites from infected mosquitoes. The FDA called chikungunya an “emerging global health threat.” (iStock)
“Infection with chikungunya virus can lead to severe disease and prolonged health problems, particularly for older adults and individuals with underlying medical conditions,” said Peter Marks, M.D., PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a press release on Thursday.
COVID-19, FLU AND RSV VACCINES ARE ALL AVAILABLE THIS FALL: SEE WHAT SOME DOCTORS RECOMMEND AND WHY
“Today’s approval addresses an unmet medical need and is an important advancement in the prevention of a potentially debilitating disease with limited treatment options,” he also said.
Before the FDA’s approval, the vaccine’s safety was tested in clinical trials that included 3,500 adults.
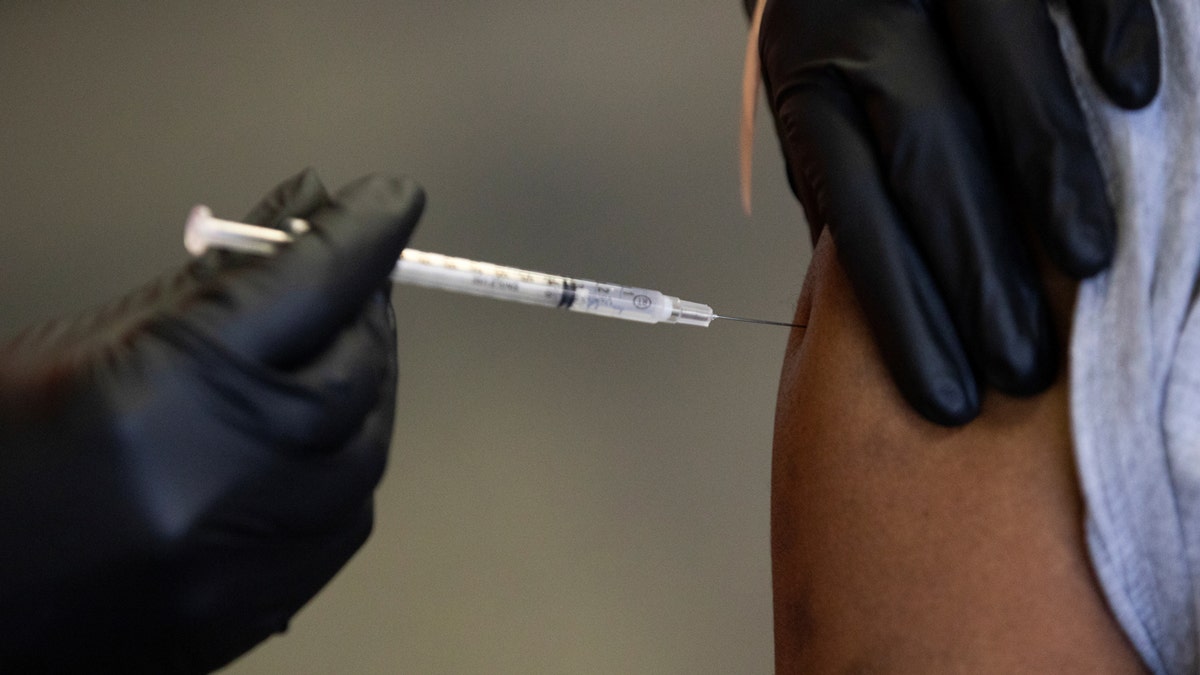
The Ixchiq vaccine is a single-dose injection that contains a weakened form of the virus. (REUTERS/Emily Elconin/File photo)
Participants most commonly reported headache, muscle pain, fatigue, joint pain, nausea, fever and tenderness at the injection site as side effects.
A small share of recipients (1.6%) experienced adverse reactions, with two of the recipients needing to be hospitalized, per the FDA’s release.
In a separate study, the vaccine’s efficacy was measured based on the immune response data of 266 adult participants.
Almost all of them were shown to have protective antibody levels.
Symptoms of the chikungunya virus
The most common symptoms are fever and joint pain, with some people also experiencing headache, muscle pain, joint swelling or rash, according to the Centers for Disease Control and Prevention (CDC).
IS IT JUST A MOSQUITO BITE — OR COULD IT BE ‘SKEETER SYNDROME’? HERE’S WHAT TO KNOW
Symptoms usually begin within three to seven days after transmission.
Most people who contract the virus get better within a week.
In rare cases, the virus can cause severe and long-lasting joint pain.

Signage is seen outside the Food and Drug Administration (FDA) headquarters in White Oak, Maryland, on Aug. 29, 2020. The agency on Thursday announced its approval of Ixchiq, the first chikungunya vaccine. (REUTERS/Andrew Kelly/File Photo)
Those at highest risk for adverse health effects include older adults, newborns who contract the infection at birth, and people with heart disease, diabetes or high blood pressure, per the CDC.
Deaths from the virus are very rare.
Location of outbreaks
Mosquitoes carrying the chikungunya virus are endemic in Africa, Southeast Asia and parts of the Americas, the FDA stated in its release.
Before 2013, cases of the chikungunya virus were primarily documented in Africa, Asia, Europe, and the Indian and Pacific Oceans.
DENGUE FEVER: WHAT YOU NEED TO KNOW ABOUT THE MOSQUITO-BORNE ILLNESS SWEEPING JAMAICA
In late 2013, the first local cases were documented in Caribbean countries, which then led to the virus spreading throughout the Americas, the CDC stated.
Treatment and prevention
For those who have been exposed and have symptoms, a blood test can confirm the presence of chikungunya or other similar viruses.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
People who are infected and experience symptoms should rest, stay hydrated with fluids and take over-the-counter medications, such as acetaminophen or paracetamol, to relieve and reduce fever, according to the CDC.
People who are traveling to countries where the virus is prevalent can reduce their risk by using insect repellent, wearing long-sleeved shirts and pants, and staying indoors or in screened areas.

People who are traveling to countries where the virus is prevalent can reduce their risk by using insect repellent, wearing long-sleeved shirts and pants, and staying indoors or in screened areas. (iStock)
Siegel noted that the vaccine — which he deems “safe and effective” — contains a live weakened version of the virus vaccine.
“That means it is not intended for [the] immunocompromised, but it is useful for those at risk of severe cases of chikungunya,” he told Fox News Digital.
CLICK HERE TO GET THE FOX NEWS APP
Added the doctor, “The vaccine is being fast-tracked, which for me means I would give it to those most at risk first, while watching post-marketing studies over next year.”
For more Health articles, visit www.foxnews.com/health.







